Home » Therapeutic Expertise » Acute Pain » Bunionectomy
Bunionectomy
Bunionectomy is a highly standardized, rapidly recruiting boney pain model.
Lotus Clinical Research takes pride in its national recognition for expertise and proficiency in bunionectomy research. As a CRO with affiliated site network, we offer comprehensive services tailored to bunionectomy trials.
Our capabilities extend to single-center studies conducted within our own facilities, ensuring standardized and efficient recruitment. When a multicenter approach is required, we leverage our extensive network of carefully selected bunionectomy sites, strategically located across diverse geographic regions.
A key factor in the success of bunionectomy research lies in minimizing variability and placebo response. To address this, Lotus Clinical Research utilizes the subject placebo response education toolkit, a powerful resource available through our CRO and site services. This toolkit has been proven effective in enhancing study outcomes and is deployed seamlessly whether we serve as the single bunionectomy center or as the CRO in multicenter trials.
By partnering with Lotus Clinical Research, you gain access to our specialized knowledge and resources in bunionectomy research. We are dedicated to ensuring rigorous study protocols, reducing variability, and optimizing results, ultimately advancing the understanding and treatment of bunionectomy-related boney pain.
Efforts are made to reduce variability among subjects in multicenter experiments by homogenizing the:
- Surgical techniques: first metatarsal Austin bunionectomy without collateral procedures
- Anesthetic technique: propofol sedation with mayo block; procedure performed with or without popiteal block depending on protocol requirements
- Recovery room procedures: standardized analgesic regimens, avoidance of ice, ambulation disallowed
- Inpatient stay: private rooms, comingling discouraged, restricted ambulation, no visitation from family or friends.
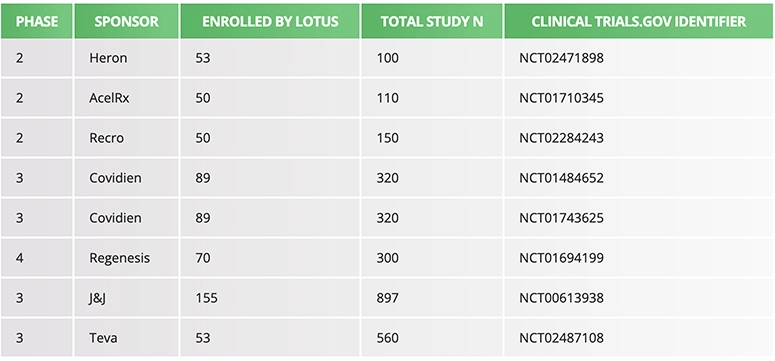
Enrollment
Average enrollment is 30 randomized subjects per month at the Lotus Clinical Research site. Screen failure rates are typically low, but are dependent on the specifics of the clinical protocol and its inclusion/exclusion criteria.